MIMICS 2
Prospective, Multi-Center International IDE Study
- PIs: Timothy M. Sullivan, MD, Thomas Zeller, MD and Masato Nakamura, MD
- Core laboratories: ultrasound; angiography; X-ray Clinical Event Committee (CEC) adjudication
- 3-year follow up
- 43 Investigational sites: US (31 sites), Germany (6 sites) and Japan (6 sites)
Baseline Patient Demographics
Enrolled population N=271
Gender male – (180/271)
Age – Mean years ± SD (N) – 68.4 ± 9.5
Diabetes Mellitus – (123/271)
Ankle Brachial Index – Mean ± SD (N) 0.7 ± 0.2 (257)
Smoker current – (219/271)
Rutherford Category
Claudication – (256/271)
CLTI – (15/271)
⇒ CLTI present in 6% of enrolled subjects
Baseline Procedural Data
Lesion Length (mm) Mean ± SD 81.2 ± 38.4 (269/271)
Total Occlusion 30.0 (81/270)
Calcification Moderate – Severe 45.9 (124/270)
⇒ Moderate – Severe Calcification present in 46% of enrolled subjects
Index Procedure Data
Stented Segment Length Mean ± SD (mm) 112.3 ± 36.3
Lesions treated with >1 BioMimics 3D stent 12.5% (34/271)
Device Success¹ 100% (271/271)
Technical Success² 100% (269/269)
1. Device Success: Successful delivery of System; placement of stent and retrieval of System
2. Technical Success: Core Lab determined ≤50% residual diameter stenosis (in-stent) at end of index procedure
⇒ More than 1 stent used in 13% of lesions
MIMICS-3D European Registry Results
Primary Endpoint – 30-Day Safety (270/271) Freedom from MAE
Primary Endpoint – 12-month Effectiveness (241/271) Freedom from CDTLR
Results
12-Months KM Freedom from loss of Primary Patency*
36-Month KM Freedom from CDTLR
Stent fracture – (0/271)
* The primary effectiveness endpoint of the MIMICS-2 Study was defined as the primary stent patency rate at 12 months
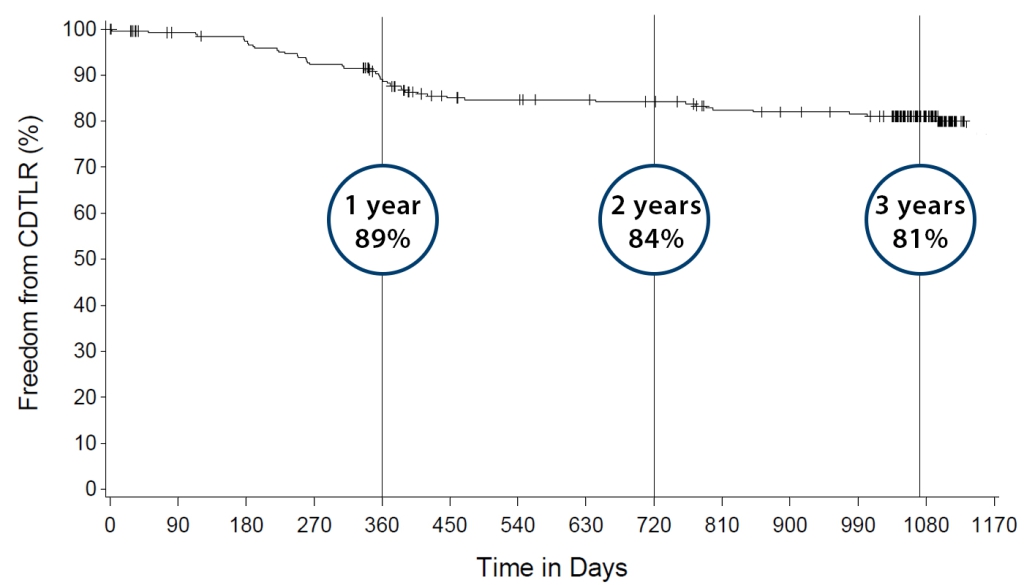
Kaplan-Meier Freedom from CDTLR over 3 years
Summary
MIMICS-2 shows continuing benefit of the BioMimics 3D Vascular Stent System at 3 Years, even in challenging cases:
- Reproducible, rigorous, high quality data from US, Japan and Europe.
- KM freedom from loss of primary patency – 83% at 1 year
- KM freedom from CDTLR – 81% at 3 years.
- Comparable outcomes to DES/DCB despite more challenging lesions and without the need for lesion preparation.
- Core Lab X-ray imaging review confirmed 0% stent fracture at 3 years.
- Providing ease-of use simplicity and long-term benefits
Data on file at Veryan Medical
All percentages are rounded to a whole number