MIMICS RCT
A randomised study comparing safety and effectiveness of the BioMimics 3D Vascular Stent
System to a straight stent control.
- PI: Thomas Zeller, MD
- Core laboratories: ultrasound; angiography; X-ray Clinical Event Committee (CEC) adjudication
- 2-year follow up
- 8 German investigational sites
- 76 patients enrolled with a 2:1 randomisation
Baseline Patient Demographics
- BioMimics 3D enrolled population N=50
- Control Stent enrolled population N=26
BioMimics 3D
Control Stent
P value
Gender male – (33/50)
Gender male – (17/26)
1.0
Age – Mean years ± SD (N) – 68 ± 10.4
Age – Mean years ± SD (N) – 67 ± 8.9
0.66
Rutherford Category
BioMimics 3D
Control Stent
P value
Rutherford category 1 – (3/50)
Rutherford category 1 – (1/26)
1.00
Rutherford category 2 – (7/50)
Rutherford category 2 – (1/26)
0.74
Rutherford category 3 – (37/50)
Rutherford category 3 – (23/26)
0.27
CLTI – (3/50)
CLTI – (1/26)
1.00
⇒ [Insert comment]
Lesion Characteristics
BioMimics 3D
Control Stent
P value
Lesion Length (mm) – 66 ± 29
Lesion Length (mm) – 63 ± 28
0.66
Occlusion (Total) – 44%
Occlusion (Total) – 46%
1.00
Calcification (Moderate to Severe) – 52%
Calcification (Moderate to Severe) – 58%
0.81
⇒ [Insert comment]
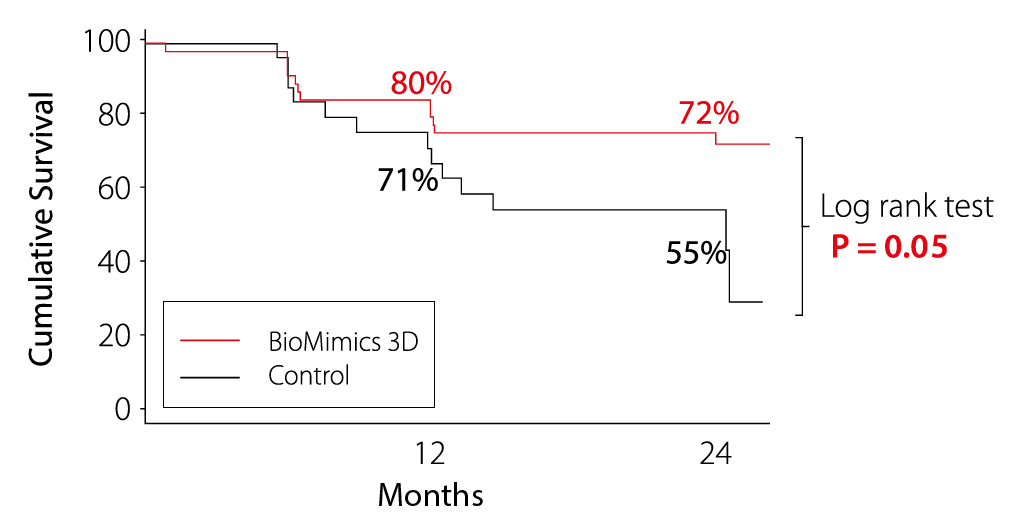
Primary Patency
Kaplan Meier Survival Estimate from Loss of Patency
Defined as PSVR >2.0, or where angiography reveals >50% diameter stenosis; or adjudicated clinically-driven TLR
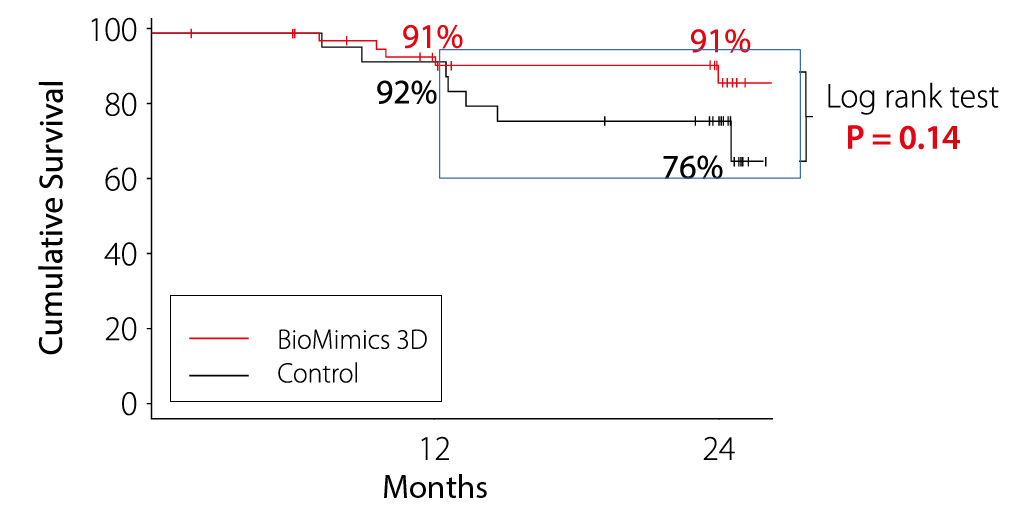
Clinically-Driven Target Lesion Revascularisation
Kaplan Meier Estimate of Survival from clinically-driven TLR determined through Independent Event Adjudication
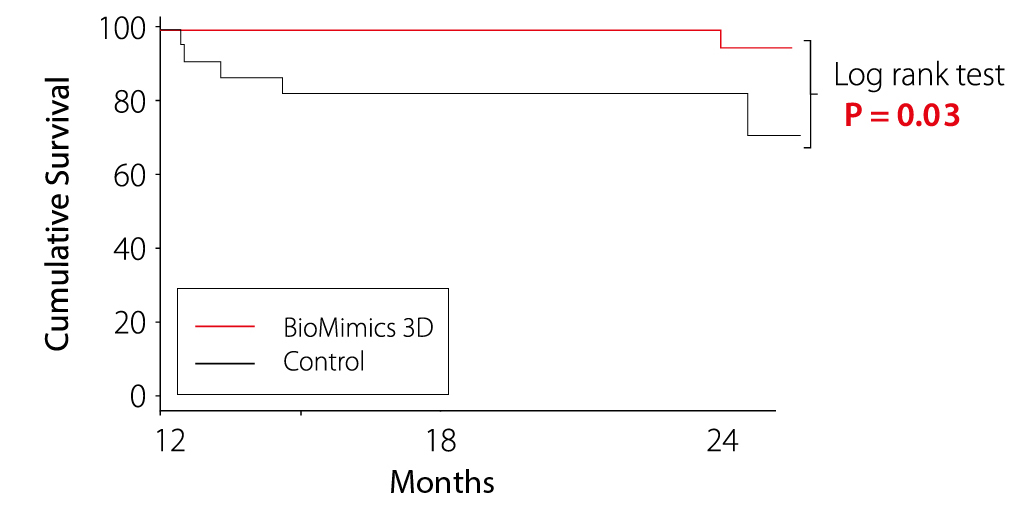
Long Term TLR Benefit
12-Month Landmark Analysis
Kaplan Meier Estimate of Survival from clinically-driven TLR determined through Independent Event Adjudication
Summary
MIMICS-RCT Provided the first clinical proof supporting the durable outcome benefit arising from the BioMimics 3D stent compared to a straight nitinol stent
- Freedom from loss of Primary Patency at 2 years
- 72% for BioMimics 3D vs 55% for straight control stents (P=0.05).
- Freedom from CDTLR
- 91% for BioMimics 3D maintained out to 2 years.
- Core lab X-ray imaging review confirmed 0% stent fractures at 2 years.
- Improvement in Rutherford Category
- 88% of patients treated with BioMimics 3D experienced an improvement of one or more Rutherford category at 2 years vs baseline.
Data on file at Veryan Medical
All percentages are rounded to a whole number