MIMICS 3D European Registry
A Prospective, Multicentre Observational Study to Evaluate BioMimics 3D Stent in PAD in the Real World
- PI: Michael Lichtenberg, MD
- Independent Clinical Event Committee (adjudication)
- 3-year follow up
- 23 Investigational sites
- 507 patients
Baseline Patient Demographics
Enrolled population N=507
Gender male – (332/507)
Age – Mean years ± SD (N) – 70 ± 10
Diabetes Mellitus – (187/507)
Ankle Brachial Index – Mean ± SD (N) 0.6 ± 0.3 (417)
Smoker current – (191/507)
Rutherford Category
Claudication – (383/504)
CLTI – (121/504)
⇒ CLTI present in 24% of enrolled subjects
Baseline Procedural Data
Diameter Stenosis – Pre-stent % ± SD 95% ± 8.0
Diameter Stenosis – Post-stent % ± SD 6% ± 8.7
Other Target Lesion Treatments Atherectomy –(39/518)
Other Target Lesion Treatments Drug Coated Ballon – (259/518)
⇒ 50% of the lesions were treated with BioMimics 3D
and Drug Coated Balloon
Baseline Patient Demographics
Stented Segment Length Mean ± SD (mm) 131 ± 80.1
1 stent – (395/518)
2-4 stents – (123/518)
⇒ More than 1 stent used in 25% of lesions
MIMICS-3D European Registry Results
Technical Success (procedure)
Procedural Success
Primary Endpoint – 30-Day Safety (486/493) Freedom from MAE
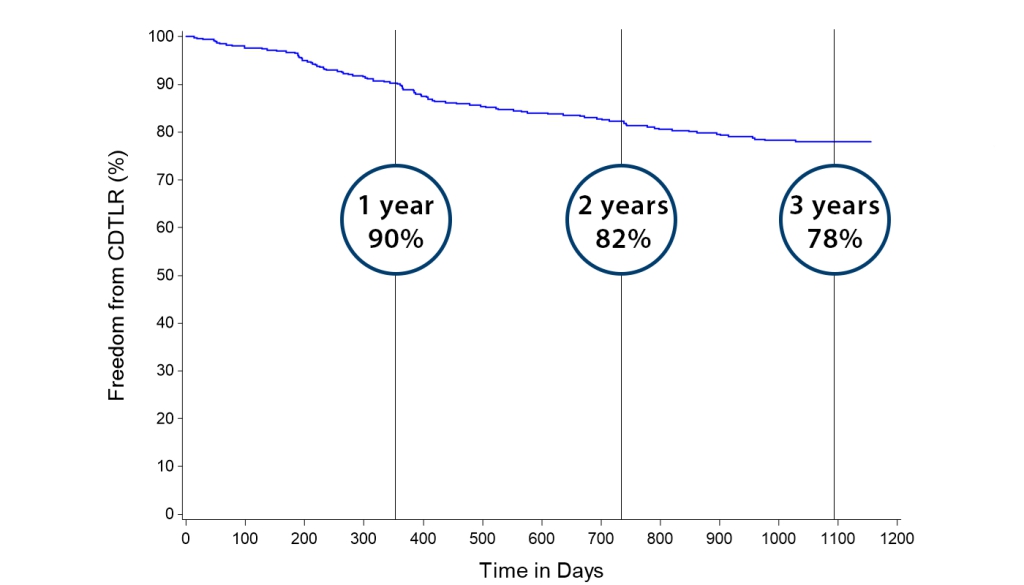
Primary Endpoint – 12-month Effectiveness (399/448) Freedom from CDTLR
3-Year Results
36-Month KM Freedom from loss of Primary Patency
36-Month KM Freedom from CDTLR
Stent fracture – (3/676)
Kaplan-Meier Freedom from CDTLR over 3 years
Comparison of KM freedom from CDTLR with
and without DCB
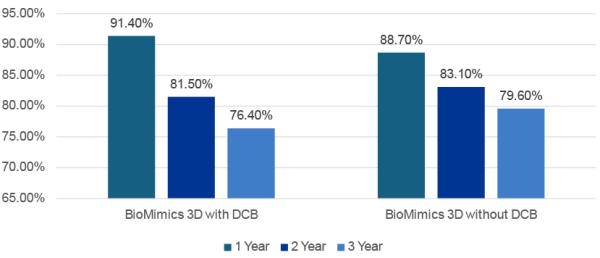
⇒ No statistical difference in CDTLR between DCB
and no-DCB cohorts
MIMICS Clinical Program
Enrolling progressively longer and more complex lesions
| Mean ± SD (mm) | MIMICS-RCT | MIMICS-2 | MIMICS-3D |
|---|---|---|---|
| Lesion Length | 66 ± 29 | 81 ± 38 | 126 ± 91 |
| Stented Segment Length | 99 ± 30 | 112 ± 36 | 131 ± 80 |
| CTO | 44% | 30% | 57% |
| Mod/Severe Ca++ | 52% | 46% | 53% |
| CLTI | 6% | 6% | 24% |
Summary
MIMICS Clinical Program investigations into the performance of the BioMimics 3D Vascular Stent System support the hypothesis that imparting non-planar curvature to the femoropopliteal artery to promote swirling blood flow and increase wall shear stress, results in clinical outcomes that are comparable to those of drug-coated and drug-eluting devices.
Data on file at Veryan Medical
All percentages are rounded to a whole number