The MIMICS Clinical Programme:
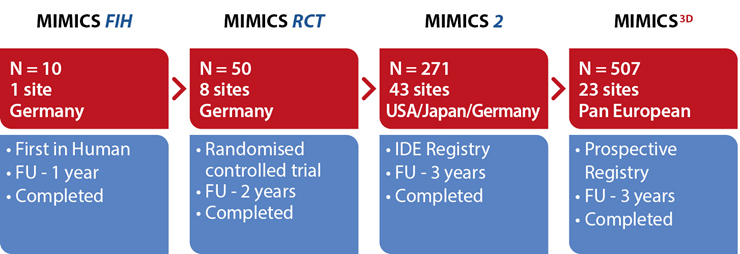
An evolving database of the safety and effectiveness of the BioMimics 3D Vascular Stent System. Gathering clinical evidence from a “real world” patient population from single de novo to complex, long and severely calcified lesions.
MIMICS-RCT
A randomized study comparing safety and effectiveness of the BioMimics 3D Vascular Stent System to a straight control stent. The difference in freedom from loss of primary patency was statistically significant through 2 years, favouring the BioMimics 3D Vascular Stent System (72% vs 55% at two years, log-rank test P=0.05). Between 12 and 24 months, there were no additional cases of CDTLR in patients with the BioMimics-3D Vascular Stent System (91% at1 & 2 years), while the number of cases of CDTLR with the control straight stent increased threefold (log-rank test P=0.03)1. There were no stent fractures at 2 years.2
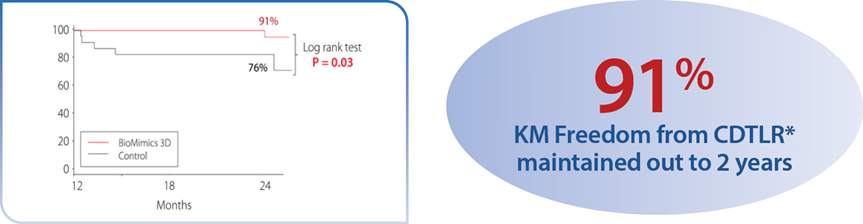
*CDTLR determined through event adjudication
MIMICS-2
A multicentre, international (USA, Japan and Germany) IDE study. At 3 years follow-up BioMimics 3D demonstrated continuing benefit with CDTLR showing comparable outcomes to DES/DCB. Core Lab X-ray imaging review confirmed 0% stent fracture in any MIMICS-2 subject treated with BioMimics.
MIMICS-2 represents a more challenging patient population than in DES/DCB pivotal trials.4,5
Continuing benefit at 3 years even in challenging cases6

MIMICS-3D
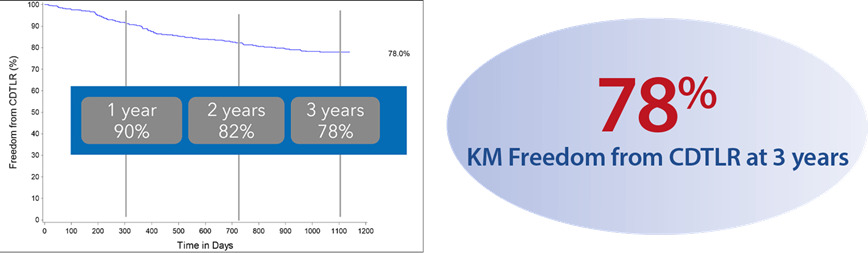
A prospective observational registry evaluating the BioMimics 3D Vascular Stent System in a real-world clinical population with a dedicated subgroup analysis of device performance as a complementary treatment in procedures involving drug-coated balloons. MIMICS-3D enrolled 507 patients across 23 clinical sites in Europe.
KM Freedom from CDTLR at 3 years7
1. Data on file at Veryan Medical treatment in procedures involving drug-coated balloons.
2. Zeller T. et al; Circ Cardiovasc Interv. 2016;9
3. Sullivan TM et al; Int J Vasc Med. 2018
4. Kenneth Rosenfield et al: N Engl J Med 2015;373:145-53. DOI: 10.1056/NEJMoa1406235
5. Michael D. Dake et al: Circ Cardiovasc Interv. 2011;4:495-504
6. Data on file at Veryan Medical – MIMICS 2 Clinical Study Report
7. Data on file at Veryan Medical – MIMICS 3D Clinical Study Report