Revolution™ Clinical Data
REVOLUTION™ REVEAL Trial
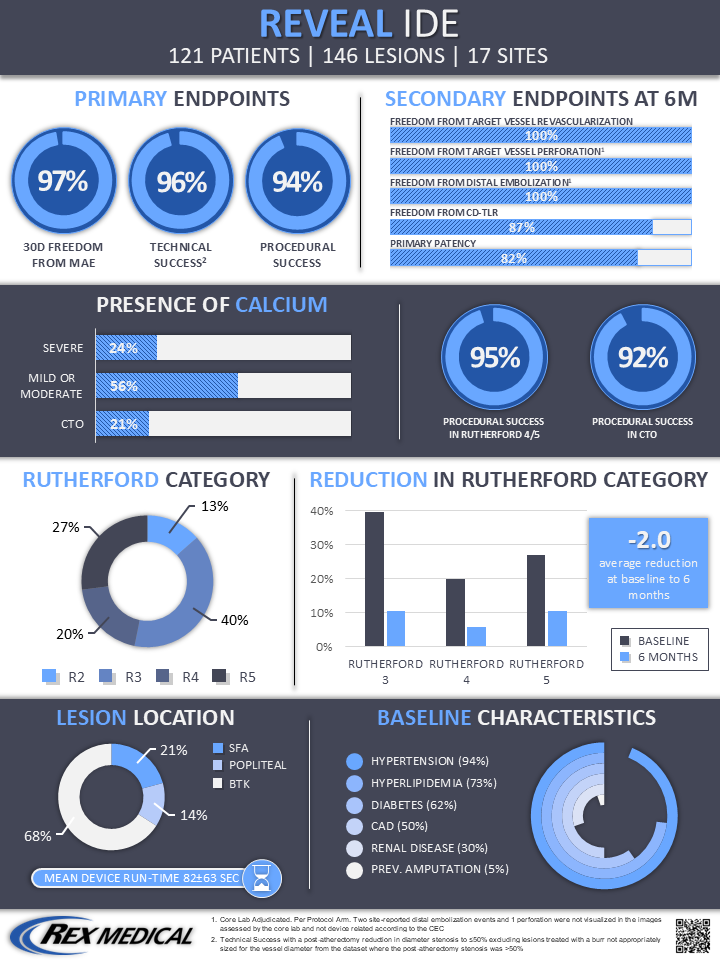
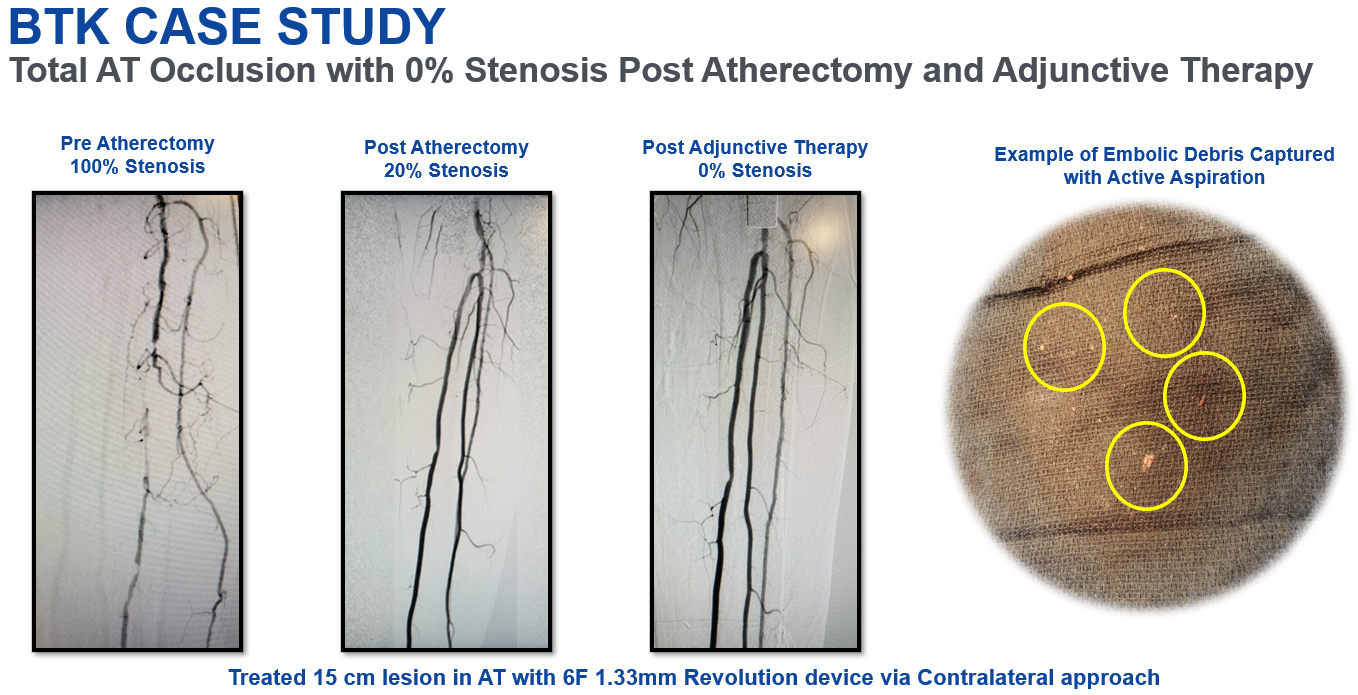
The REVEAL Investigational Device Exemption (IDE) study evaluated the safety and effectiveness of lower extremity arterial atherectomy with the Revolution™ Peripheral Atherectomy System device in 121 ITT subjects with 146 target lesions. The findings confirm a favorable safety and effectiveness profile through 6 months for the Rex Medical Revolution™ Peripheral Atherectomy System when used in subjects with symptomatic infrainguinal arterial occlusive disease.